 cochrane评分表.docx
cochrane评分表.docx
- 文档编号:9968716
- 上传时间:2023-02-07
- 格式:DOCX
- 页数:12
- 大小:19.51KB
cochrane评分表.docx
《cochrane评分表.docx》由会员分享,可在线阅读,更多相关《cochrane评分表.docx(12页珍藏版)》请在冰豆网上搜索。
cochrane评分表
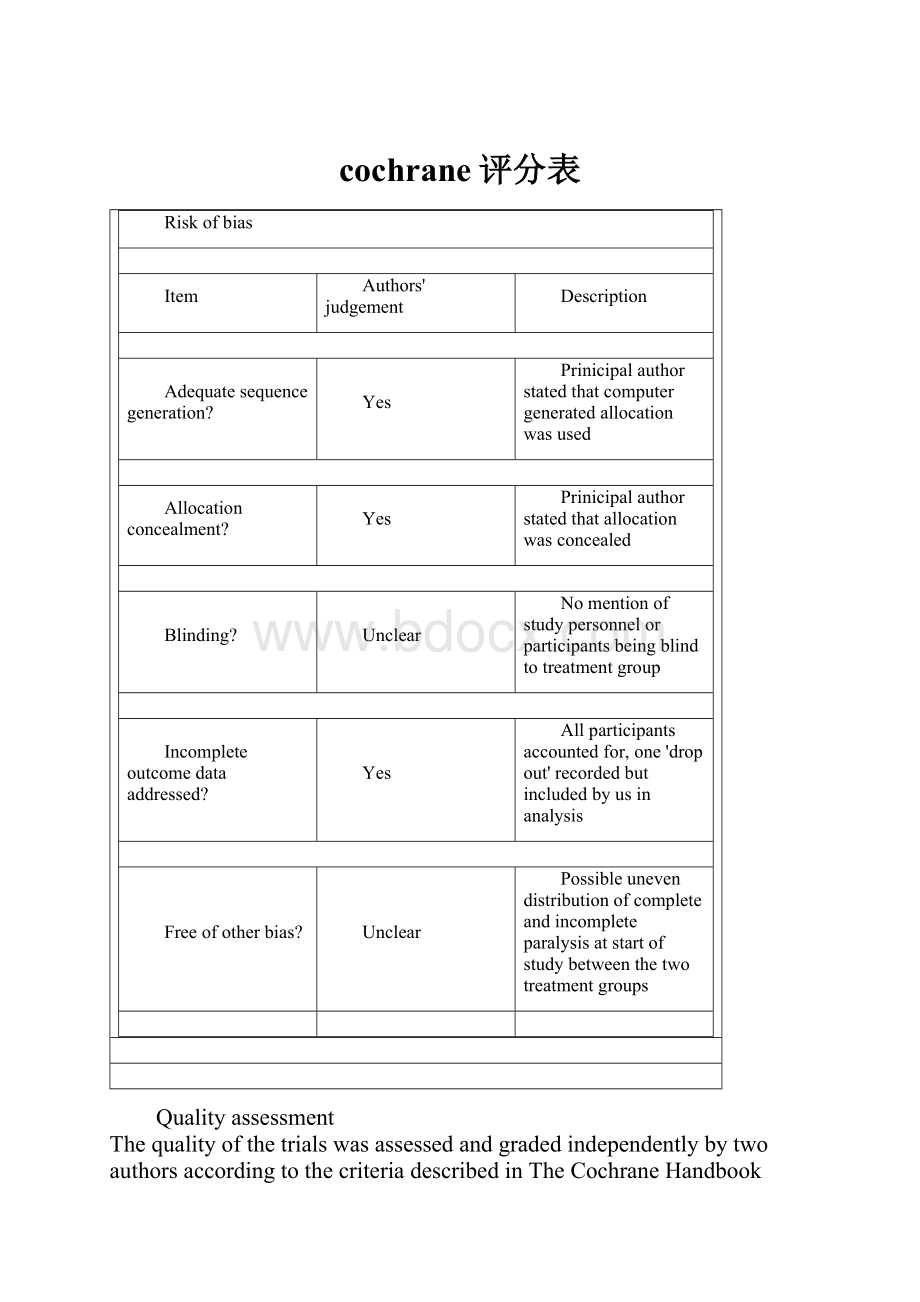
Riskofbias
Item
Authors'judgement
Description
Adequatesequencegeneration?
Yes
Prinicipalauthorstatedthatcomputergeneratedallocationwasused
Allocationconcealment?
Yes
Prinicipalauthorstatedthatallocationwasconcealed
Blinding?
Unclear
Nomentionofstudypersonnelorparticipantsbeingblindtotreatmentgroup
Incompleteoutcomedataaddressed?
Yes
Allparticipantsaccountedfor,one'dropout'recordedbutincludedbyusinanalysis
Freeofotherbias?
Unclear
Possibleunevendistributionofcompleteandincompleteparalysisatstartofstudybetweenthetwotreatmentgroups
Qualityassessment
ThequalityofthetrialswasassessedandgradedindependentlybytwoauthorsaccordingtothecriteriadescribedinTheCochraneHandbook4.2.6(Higgins2006).Gradingswerecomparedandanyinconsistenciesbetweentheauthorsintheinterpretationofinclusioncriteriaandtheirsignificancetotheselectedstudywerediscussedandresolved.
Theselectedstudywasassessedforthefollowingcharacteristics:
1.Theadequacyoftherandomisationprocess(possibleselectionbias).Adequaterandomisationincludesanyoneofthefollowingmethods:
computergeneratedortableofrandomnumbers,drawingoflots,coin-toss,shufflingcardsorthrowofadice.Inadequatemethodsofrandomisationincludethefollowing:
caserecordnumber,dateofbirthoralternatenumbers.
2.Theadequacyoftheallocationconcealment(possibleselectionbias).Adequatemethodsofallocationconcealmentincludeeithercentralrandomisation(i.e.separatetootheraspectsoftrialadministration)orsequentiallynumberedsealedopaqueenvelopes.Inadequateconcealmentmeansanopenallocationsequenceinwhicheitherparticipantsortrialistswereabletoforeseetheupcomingassignment.
3.Theblindingofoutcomeassessors(i.e.whetherthepersonsassessingtheoutcomeofcarewereawareofwhichtreatmenttheparticipanthadreceived-possibleperformancebias).
4.Theextentandhandlingoflossestofollowup(possibleattritionbias).Adequatehandlingoflossestofollowupinvolvesacleardescriptionandexplanationbeinggivenofanysignificantdifferencebetweenthelossesoftheinterventiongroups.Anunacceptablelossinanyoneinterventiongroupwasconsideredtobelossgreaterthan20%.
StudygradingsA,BorCwereemployedforoverallqualityasfollows.
A:
Minimisationofbiasinallfourcategoriesabove:
i.e.adequaterandomisation,fewlossestofollowupandintention-to-treatanalysis,blindingofoutcomeassessors,highqualityoutcomeassessment;
B:
EachofthecriteriainApartiallymet;
C:
OneormoreofthecriteriainAnotmet.
Riskofbiasinincludedstudies
WeclassifiedthisstudyasgradeCbecauseoftheuncertaintyaboutblinding.Thepossibilityofanunevendistributionofcompleteandincompletepalsiesbetweenthetwogroupsisanotherpotentialsourceofbiasandweconcludeoverallthatthisisalowqualitystudy.
Table8.5.a:
TheCochraneCollaboration’stoolforassessingriskofbias
Domain
Description
Reviewauthors’judgement
Sequencegeneration.
Describethemethodusedtogeneratetheallocationsequenceinsufficientdetailtoallowanassessmentofwhetheritshouldproducecomparablegroups.
Wastheallocationsequenceadequatelygenerated?
Allocationconcealment.
Describethemethodusedtoconcealtheallocationsequenceinsufficientdetailtodeterminewhetherinterventionallocationscouldhavebeenforeseeninadvanceof,orduring,enrolment.
Wasallocationadequatelyconcealed?
Blindingofparticipants,personnelandoutcomeassessorsAssessmentsshouldbemadeforeachmainoutcome(orclassofoutcomes).
Describeallmeasuresused,ifany,toblindstudyparticipantsandpersonnelfromknowledgeofwhichinterventionaparticipantreceived.Provideanyinformationrelatingtowhethertheintendedblindingwaseffective.
Wasknowledgeoftheallocatedinterventionadequatelypreventedduringthestudy?
IncompleteoutcomedataAssessmentsshouldbemadeforeachmainoutcome(orclassofoutcomes).
Describethecompletenessofoutcomedataforeachmainoutcome,includingattritionandexclusionsfromtheanalysis.Statewhetherattritionandexclusionswerereported,thenumbersineachinterventiongroup(comparedwithtotalrandomizedparticipants),reasonsforattrition/exclusionswherereported,andanyre-inclusionsinanalysesperformedbythereviewauthors.
Wereincompleteoutcomedataadequatelyaddressed?
Selectiveoutcomereporting.
Statehowthepossibilityofselectiveoutcomereportingwasexaminedbythereviewauthors,andwhatwasfound.
Arereportsofthestudyfreeofsuggestionofselectiveoutcomereporting?
Othersourcesofbias.
Stateanyimportantconcernsaboutbiasnotaddressedintheotherdomainsinthetool.
Ifparticularquestions/entrieswerepre-specifiedinthereview’sprotocol,responsesshouldbeprovidedforeachquestion/entry.
Wasthestudyapparentlyfreeofotherproblemsthatcouldputitatahighriskofbias?
Table8.5.c:
Criteriaforjudgingriskofbiasinthe‘Riskofbias’assessmenttool
SEQUENCEGENERATION
Wastheallocationsequenceadequatelygenerated?
[Shortform:
Adequatesequencegeneration?
]
Criteriaforajudgementof‘YES’(i.e.lowriskofbias).
Theinvestigatorsdescribearandomcomponentinthesequencegenerationprocesssuchas:
∙Referringtoarandomnumbertable;
∙Usingacomputerrandomnumbergenerator;
∙Cointossing;
∙Shufflingcardsorenvelopes;
∙Throwingdice;
∙Drawingoflots;
∙Minimization*.
*Minimizationmaybeimplementedwithoutarandomelement,andthisisconsideredtobeequivalenttobeingrandom.
Criteriaforthejudgementof‘NO’(i.e.highriskofbias).
Theinvestigatorsdescribeanon-randomcomponentinthesequencegenerationprocess.Usually,thedescriptionwouldinvolvesomesystematic,non-randomapproach,forexample:
∙Sequencegeneratedbyoddorevendateofbirth;
∙Sequencegeneratedbysomerulebasedondate(orday)ofadmission;
∙Sequencegeneratedbysomerulebasedonhospitalorclinicrecordnumber.
Othernon-randomapproacheshappenmuchlessfrequentlythanthesystematicapproachesmentionedaboveandtendtobeobvious. Theyusuallyinvolvejudgementorsomemethodofnon-randomcategorizationofparticipants,forexample:
∙Allocationbyjudgementoftheclinician;
∙Allocationbypreferenceoftheparticipant;
∙Allocationbasedontheresultsofalaboratorytestoraseriesoftests;
∙Allocationbyavailabilityoftheintervention.
Criteriaforthejudgementof‘UNCLEAR’(uncertainriskofbias).
Insufficientinformationaboutthesequencegenerationprocesstopermitjudgementof‘Yes’or‘No’.
ALLOCATIONCONCEALMENT
Wasallocationadequatelyconcealed?
[Shortform:
Allocationconcealment?
]
Criteriaforajudgementof‘YES’(i.e.lowriskofbias).
Participantsandinvestigatorsenrollingparticipantscouldnotforeseeassignmentbecauseoneofthefollowing,oranequivalentmethod,wasusedtoconcealallocation:
∙Centralallocation(includingtelephone,web-based,andpharmacy-controlled,randomization);
∙Sequentiallynumbereddrugcontainersofidenticalappearance;
∙Sequentiallynumbered,opaque,sealedenvelopes.
Criteriaforthejudgementof‘NO’(i.e.highriskofbias).
Participantsorinvestigatorsenrollingparticipantscouldpossiblyforeseeassignmentsandthusintroduceselectionbias,suchasallocationbasedon:
∙Usinganopenrandomallocationschedule(e.g.alistofrandomnumbers);
∙Assignmentenvelopeswereusedwithoutappropriatesafeguards(e.g.ifenvelopeswereunsealedornonopaqueornotsequentiallynumbered);
∙Alternationorrotation;
∙Dateofbirth;
∙Caserecordnumber;
∙Anyotherexplicitlyunconcealedprocedure.
Criteriaforthejudgementof‘UNCLEAR’(uncertainriskofbias).
Insufficientinformationtopermitjudgementof‘Yes’or‘No’.Thisisusuallythecaseifthemethodofconcealmentisnotdescribedornotdescribedinsufficientdetailtoallowadefinitejudgement–forexampleiftheuseofassignmentenvelopesisdescribed,butitremainsunclearwhetherenvelopesweresequentiallynumbered,opaqueandsealed.
BLINDINGOFPARTICIPANTS,PERSONNELANDOUTCOMEASSESSORS
Wasknowledgeoftheallocatedinterventionsadequatelypreventedduringthestudy?
[Shortform:
Blinding?
]
Criteriaforajudgementof‘YES’(i.e.lowriskofbias).
Anyoneofthefollowing:
∙Noblinding,butthereviewauthorsjudgethattheoutcomeandtheoutcomemeasurementarenotlikelytobeinfluencedbylackofblinding;
∙Blindingofparticipantsandkeystudypersonnelensured,andunlikelythattheblindingcouldhavebeenbroken;
∙Eitherparticipantsorsomekeystudypersonnelwerenotblinded,butoutcomeassessmentwasblindedandthenon-blindingofothersunlikelytointroducebias.
Criteriaforthejudgementof‘NO’(i.e.highriskofbias).
Anyoneofthefollowing:
∙Noblindingorincompleteblinding,andtheoutcomeoroutcomemeasurementislikelytobeinfluencedbylackofblinding;
∙Blindingofkeystudyparticipantsandpersonnelattempted,butlikelythattheblindingcouldhavebeenbroken;
∙Eitherparticipantsorsomekeystudypersonnelwerenotblinded,andthenon-blindingofotherslikelytointroducebias.
Criteriaforthejudgementof‘UNCLEAR’(uncertainriskofbias).
Anyoneofthefollowing:
∙Insufficientinformationtopermitjudgementof‘Yes’or‘No’;
∙Thestudydidnotaddressthisoutcome.
INCOMPLETEOUTCOMEDATA
Wereincompleteoutcomedataadequatelyaddressed?
[Shortform:
Incompleteoutcomedataaddressed?
]
Criteriaforajudgementof‘YES’(i.e
- 配套讲稿:
如PPT文件的首页显示word图标,表示该PPT已包含配套word讲稿。双击word图标可打开word文档。
- 特殊限制:
部分文档作品中含有的国旗、国徽等图片,仅作为作品整体效果示例展示,禁止商用。设计者仅对作品中独创性部分享有著作权。
- 关 键 词:
- cochrane 评分
 冰豆网所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
冰豆网所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。


 1212中级汽车维修工考试试题三.docx
1212中级汽车维修工考试试题三.docx
